

Sito ufficiale di informazione scientifica e clinica sulla Scrambler Therapy®
ST-NET è una rete internazionale automatizzata ad accesso gratuito per gli utilizzatori della Scrambler Therapy®. Questa rete ha vari scopi, ma il principale è quello di supporto agli studi clinici ed ai Centri Pilota, in modo da ridurre al minimo la variabilità dei risultati connessi alla componente operatore dipendente. In questo modo si crea un sistema di qualità che indipendentemente dalla tipologia di studio clinico scelta dal ricercatore, garantisce una ripetibilità ed una coerenza di risultati altrimenti non ottenibile. Analogamente avviene per le prestazioni dei Centri Pilota, che non potrebbero essere definiti tali in assenza di un sistema di qualità specifico per questa metodica.
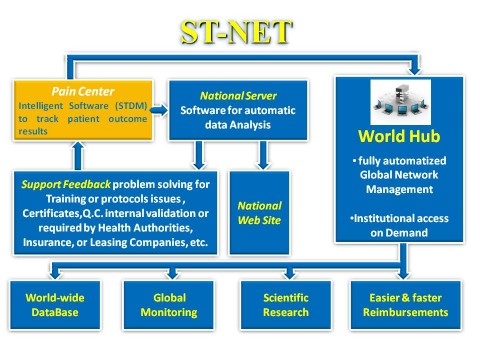 La cartella
clinica basata sul software STDM (Scrambler Therapy Data Manager)
raccoglie i dati dei trattamenti in un database visuale di facile
utilizzo. Durante la compilazione avvisa in automatico l’operatore
di eventuali criticità riscontrate, suggerisce i protocolli di
trattamento più adatti al tipo di paziente in cura, permette di
utilizzare strumenti idonei per la ricerca come il Brief Pain
Inventory, Pain Detect, DN4 e molto altro ancora. Questo software
può aprire istantaneamente (anche mentre si lavora nella cartella)
i dati del paziente in altri software tradizionali come Excel,
Word, o più semplicemente in Notepad. E’ anche possibile
analizzare l’andamento del trattamento con grafici istantanei che
non richiedono nessuna impostazione preliminare, od esportare
l’intero Database in formati standard con criteri di filtraggio
programmabili per data, tipologia di dolore, ed altre
caratteristiche significative negli studi clinici.
La cartella
clinica basata sul software STDM (Scrambler Therapy Data Manager)
raccoglie i dati dei trattamenti in un database visuale di facile
utilizzo. Durante la compilazione avvisa in automatico l’operatore
di eventuali criticità riscontrate, suggerisce i protocolli di
trattamento più adatti al tipo di paziente in cura, permette di
utilizzare strumenti idonei per la ricerca come il Brief Pain
Inventory, Pain Detect, DN4 e molto altro ancora. Questo software
può aprire istantaneamente (anche mentre si lavora nella cartella)
i dati del paziente in altri software tradizionali come Excel,
Word, o più semplicemente in Notepad. E’ anche possibile
analizzare l’andamento del trattamento con grafici istantanei che
non richiedono nessuna impostazione preliminare, od esportare
l’intero Database in formati standard con criteri di filtraggio
programmabili per data, tipologia di dolore, ed altre
caratteristiche significative negli studi clinici.
Cliccando su "Analisi automatica" un algoritmo analizza i dati del trattamento ed evidenzia i principali parametri di controllo, indicando la percentuale di scostamento dai valori normali. Scostamenti significativi o anomalie di particolare gravità vengono evidenziati immediatamente.
ST-NET è pienamente conforme agli standard sulla privacy di HIPAA e GDPR (General Data Protection Regulation).
Contatti Download Video Tutorial Delta Research & Development
Copyright © Delta Research & Development. All rights reserved