

Welcome to the Scrambler Therapy® official scientific and clinical information site
The ST-NET project is based on ST-NET, an international automated network free for Scrambler Therapy® users. This network has been set up for various purposes, but its main one is to support clinical trials and pain treatment centers in reducing to a minimum operator-dependent variability results. By so doing, a quality system is created that, regardless of clinical trials chosen by researchers, guarantees reproducibility and consistent results, which are otherwise not achievable, and therefore more reliable scientific clinical trials.
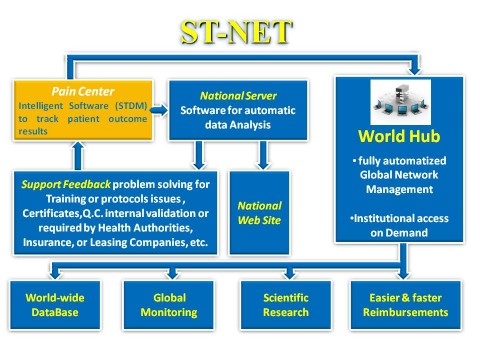 ST-NET is focused on a clinical “intelligent” medical record based
on dedicated software designed exclusively for Scrambler Therapy.
Scrambler Therapy Data Manager (STDM) software manages in a simple
and effective way patients medical records in an easy-to-use
visual database. During compilation, STDM automatically
informs the operator of possible critical features and
suggests the most fit treatment protocols for any patient and
enables the use of specific research tools such as Brief Pain
Inventory, Pain Detect, DN4, and much more. This software can
simultaneously (while you are working on the record) open
patient's data on other traditional software such as Excel, Word,
or simply Notepad. It is also possible to analyze treatment trends
with graphs that do not require previous configuration. The entire
database can be exported in Excel with filtering criteria based on
data, pain type, or other meaningful characteristics in clinical
trials.
ST-NET is focused on a clinical “intelligent” medical record based
on dedicated software designed exclusively for Scrambler Therapy.
Scrambler Therapy Data Manager (STDM) software manages in a simple
and effective way patients medical records in an easy-to-use
visual database. During compilation, STDM automatically
informs the operator of possible critical features and
suggests the most fit treatment protocols for any patient and
enables the use of specific research tools such as Brief Pain
Inventory, Pain Detect, DN4, and much more. This software can
simultaneously (while you are working on the record) open
patient's data on other traditional software such as Excel, Word,
or simply Notepad. It is also possible to analyze treatment trends
with graphs that do not require previous configuration. The entire
database can be exported in Excel with filtering criteria based on
data, pain type, or other meaningful characteristics in clinical
trials.
By clicking on “Automatic analysis,” an algorithm analyzes the treatment data and highlights the main control parameters, indicating the percentage shift from the normal values. Meaningful shifts or serious anomalies are highlighted.
ST-NET is fully compliant with HIPAA and GDPR (General Data Protection Regulation) privacy standards. Implemented multi-level protection requirements are higher than those in most popular commercial software.
Contact Download Video Tutorial Delta Research & Development
Copyright © Delta Research & Development. All rights reserved